How to Solve Spinal Endoscope Procurement Challenges? QUNaMai Platform: ISO-Certified Devices & Precise Supply-Demand Matching
Los autores: QuNaMai
hora de lanzamiento: 2025-09-02 16:35:00
número de vista: 694
How to Solve Spinal Endoscope Procurement Challenges? QUNaMai Platform: ISO-Certified Devices + Precise Supply-Demand Matching for Efficient Solutions
For spinal surgery departments and procurement offices in hospitals, spinal endoscope procurement has always faced three core challenges: first, difficulty in verifying device qualifications, with concerns about compliance risks if devices fail to meet international and domestic standards; second, poor device compatibility, as separate procurement of endoscope bodies, tools, and auxiliary equipment often leads to mismatched specifications and uncoordinated operations; third, inefficient supply-demand matching, such as difficulty in finding high-quality suppliers or high communication costs and long procurement cycles. However, QUNaMai Platform (www.tl-wc.com) — a vertical service hub focusing on medical surgical instruments — has always taken "Ensuring surgical safety and simplifying medical procurement" as its mission and "Building a global ecosystem for 'compliant connection + efficient circulation' of medical devices" as its vision. Through the model of "integrating ISO-certified devices + precise supply-demand matching", it is gradually solving these procurement pain points and becoming a preferred platform for more medical institutions to procure spinal endoscopes.
1. Dual ISO Certifications + National Standard Compliance: Fully Avoid "Compliance Risks" in Spinal Endoscope Procurement
The top priority for medical institutions when procuring spinal endoscopes is "qualification compliance" — after all, devices are directly related to surgical safety. Failure to meet standards will not only affect clinical use but also may lead to regulatory penalties. To fulfill its mission of "ensuring surgical safety", QUNaMai Platform takes "compliance" as the core screening criterion when integrating spinal endoscope resources. All displayed spinal endoscopes and supporting tools have obtained ISO 13485:2016 Medical Device Quality Management System Certification and ISO 9001:2015 Quality Management System Certification, while complying with domestic standards such as GB/T 42061-2022 (Risk Management for Medical Devices) and GB/T 19001-2016, achieving "international + domestic" dual compliance guarantees.
More thoughtfully, the platform clearly marks certification information for each spinal endoscope. For example, the platform's flagship 6.3MM System Spinal Endoscope Set (https://www.tl-wc.com/products/153) not only displays ISO certification numbers and key items of national standard test reports directly on its page but also details core parameters such as 6.3mm endoscope diameter, 30° viewing angle, and 3.75mm instrument channel. Procurers do not need to contact manufacturers separately to obtain qualification documents; they only need to click the link to enter the product details page to complete "compliance pre-verification + parameter confirmation". For procurement offices, this not only saves a lot of time in qualification review but also avoids the risk of "procuring non-compliant devices" from the source, making spinal endoscope procurement more secure and aligning with the platform's original mission of "simplifying medical procurement".
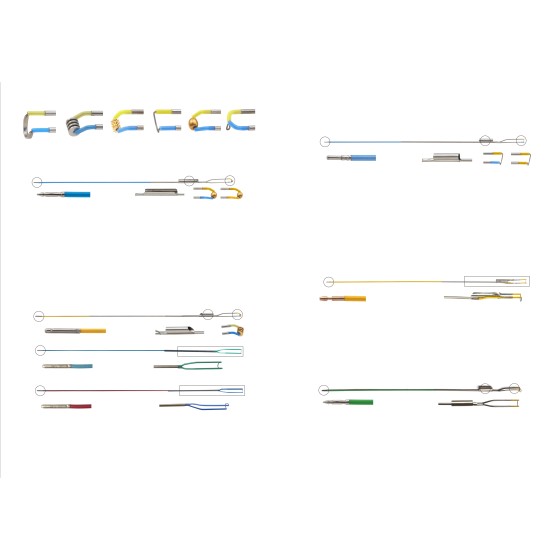
2. One-Stop Integration of "Endoscope Body + Tools + Auxiliary Equipment": Solving the "Compatibility" Challenge of Spinal Endoscopes
Spinal endoscope surgery has extremely high requirements for device compatibility: the endoscope diameter must match the surgical incision size, the nucleus pulposus forceps specifications must be suitable for operations on different parts of the cervical and lumbar spine, and auxiliary equipment (such as imaging systems and pneumatic arms) must collaborate seamlessly with the endoscope body. If devices of different brands are procured separately, problems such as "mismatch between the endoscope channel and tools" and "imaging resolution failing to keep up with the endoscope's field of view" are likely to occur, affecting surgical efficiency.
Centered on its vision of "building an efficient circulation ecosystem", QUNaMai Platform addresses this pain point by integrating end-to-end compatible sets covering the "entire spinal endoscope workflow" based on "clinical needs", completely solving the trouble of "separate procurement and poor compatibility":
- Comprehensive coverage of endoscope body specifications: In addition to the aforementioned 6.3MM System Spinal Endoscope Set (https://www.tl-wc.com/products/153), the platform also integrates spinal endoscopes with multiple diameters such as 6.9mm and 7.3mm-10.0mm, all designed with a 30° viewing angle to meet the needs of different procedures from minimally invasive small incisions to conventional surgeries. The instrument channels of all endoscope bodies are uniformly controlled between 3.75mm and 4.3mm to ensure perfect compatibility with subsequent tools;
- Tool classification based on "clinical actions": Nucleus pulposus forceps are precisely classified by "straight head/45° round cup/long cup" and "2.0mm-3.5mm specifications". For example, 2.0mm straight-head nucleus pulposus forceps are suitable for precise grasping in the cervical spine, while 3.5mm 45° round-cup nucleus pulposus forceps are suitable for lumbar nucleus pulposus removal. Each tool matches the specifications of the endoscope channel; the ligamentum flavum biting and cutting forceps are also equipped with overload protection devices to avoid operational errors during surgery, balancing compatibility and safety;
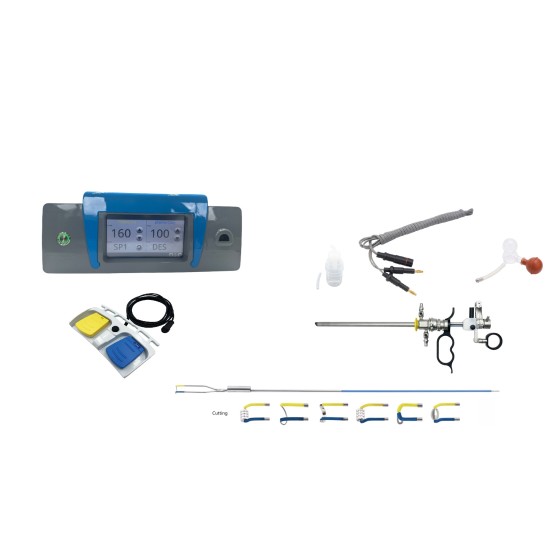
- "Scenario-based matching" of auxiliary equipment: The 4K ultra-high-definition imaging system collaborates seamlessly with all endoscope bodies to ensure clear surgical vision. Especially in UBE (Unilateral Biportal Endoscopy) surgical scenarios, the 70cm titanium steel serpentine arm pneumatic auxiliary device (https://www.tl-wc.com/products/41) integrated by the platform not only supports more than 4 surgical positions such as lateral position and prone position but also is equipped with anti-misoperation switch design. It can collaborate seamlessly with mainstream spinal endoscopes such as the 6.3MM model (https://www.tl-wc.com/products/153) to achieve "endoscope body-tools-auxiliary equipment" integrated operation, eliminating the need for procurers to "search for goods separately and test compatibility repeatedly" and greatly improving procurement efficiency.
3. Precise Supply-Demand Matching: Helping Spinal Endoscope Procurement "Avoid Detours"
In traditional spinal endoscope procurement, procurers often face problems such as "difficulty in finding goods and chaotic matching": either they do not know which suppliers have compliant products, or after contacting multiple manufacturers, they find that the products do not meet the department's needs, resulting in prolonged procurement cycles and increased communication costs. With "simplifying medical procurement" as its mission, one of QUNaMai Platform's core values is to create a closed-loop service of "ISO-certified spinal endoscopes + precise supply-demand matching", enabling procurers to "find the right goods and achieve accurate matching" and promoting the implementation of the "efficient circulation ecosystem".
Through the "Where to Buy" entry, the platform provides targeted matching services for procurers:
- If a hospital's spinal surgery department needs a "6.3MM System Spinal Endoscope Set + 4K imaging system", it only needs to click the "Where to Buy" button on the product details page (https://www.tl-wc.com/products/153). The platform will match 3-5 high-quality suppliers with ISO certification and rich clinical cases based on procurement volume (bulk procurement/spot replenishment) and regional needs, avoiding "blind goods searching" by procurers;
- If the procurement office also needs a pneumatic arm auxiliary device for supporting UBE surgery, it can also use the matching entry of (https://www.tl-wc.com/products/41) to achieve one-stop supplier matching for "main devices + auxiliary equipment", without the need to contact multiple manufacturers separately;
- Throughout the matching process, the platform will follow up closely, assist procurers in verifying supplier qualifications and confirming product compliance, ensuring "information symmetry between supply and demand sides". From "goods searching" to "matching" and then to "qualification verification", the entire process is efficient and transparent, ensuring that each procurement meets the platform's core goal of "ensuring safety and improving efficiency".
Conclusion: Choose QUNaMai for More Compliant and Efficient Spinal Endoscope Procurement
For medical institutions, spinal endoscope procurement is not just about "buying products" but also about "buying compliance, compatibility, and efficiency". Adhering to its mission of "ensuring surgical safety and simplifying medical procurement" and moving towards its vision of "building a global efficient circulation ecosystem for medical devices", QUNaMai Platform is becoming a key platform for solving spinal endoscope procurement pain points by integrating ISO-certified device resources such as the 6.3MM System Spinal Endoscope Set (https://www.tl-wc.com/products/153) and UBE pneumatic arm auxiliary device (https://www.tl-wc.com/products/41), solving device compatibility issues, and providing precise supply-demand matching services. Whether a hospital's procurement office needs to procure compliant devices in bulk or a spinal surgery department needs to supplement compatible tools, they can achieve "one-stop screening, compliant verification, and precise matching" through QUNaMai Platform and specific product links, allowing spinal endoscope procurement to avoid detours and focus more on improving clinical surgical safety and efficiency, jointly promoting the upgrading of medical service quality.
bienes relacionados

-560x560.png)
